Emerson: DeltaV Workflow Management Next-Generation Software Streamlines Pharmaceutical Development, Accelerates Speed to Market
April 16, 2024
Emerson has recently introduced DeltaV Workflow Management, a next-generation software designed for life sciences companies in early-stage development.
The new software continues Emerson’s expansion of capabilities of the evolving DeltaV automation platform to offer more scalable options to suit the needs of smaller life sciences innovators. For companies with limited IT infrastructure, DeltaV Workflow Management provides a cloud-based, software-as-a-service solution for simple recipe authoring, execution and electronic data capture.
In high-growth life sciences companies, such as those making advanced therapy medicinal products for hard-to-treat diseases and personalized medicines, manufacturing facilities still heavily rely on manual processes and record-keeping for bench-scale operations and recipe authoring. Many do not have robust IT departments, nor staff experienced in the complex coding necessary with traditional manufacturing execution systems.
DeltaV Workflow Management removes this layer of complexity by transitioning recipe workflow data from manual records to digital “paper on glass,” providing a simple and scalable solution that helps accelerate the drug development process with no coding experience required. The software also generates searchable digital records that are easily organized and exported and data that can be more easily analyzed and reported. In addition, this digital shift minimizes the contamination risks associated with paper records in sterile clean rooms.
“DeltaV Workflow Management enables innovation for both startups and established labs that demand speed and simplicity in early-stage drug development,” said Nathan Pettus, president of Emerson’s process systems and solutions business. “Recipes relying on standard, full-blown manufacturing execution systems can take years of experience and months to create and fully automate. With this new software, the timeline is reduced to days, providing companies with a head start toward commercialization and getting new therapeutic medicines into the pipeline faster.”
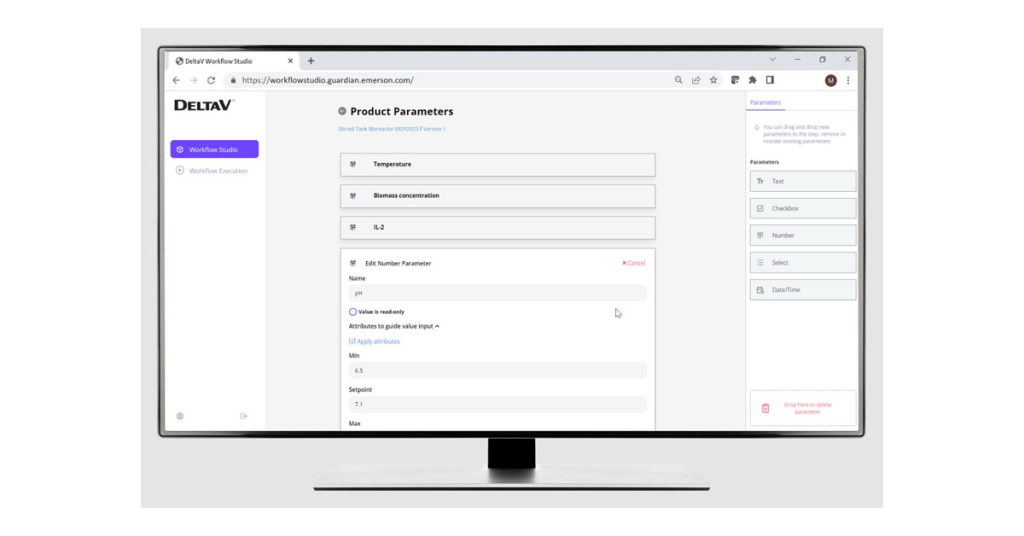
Within DeltaV Workflow Management, users employ simple drag-and-drop elements to create steps for a recipe and automate workflows that meet Good Manufacturing Practice standards. Within those steps, they can add new parameters and e-signature requirements for quality control and regulatory compliance, run product recipes, authorize users for specific tasks and create digital batch records.
DeltaV Workflow Management expands and complements the user options for digital recipe development and execution already provided by Emerson’s DeltaV MES—the newest evolution of the Syncade MES. Users now have additional flexibility to match digital recipe solutions to their business needs, underscoring Emerson’s commitment to continuing enhancement of and investment in a portfolio of manufacturing execution solutions for organizations at any scale.