Yokogawa Releases OpreX Environmental Monitoring System for Pharmaceutical and Medical Device Industries
October 26, 2020
Yokogawa Electric Corporation recently announced the October 23 global release of the OpreX Environmental Monitoring System for the pharmaceutical and medical device industries. The new product is a data collection and recording system that records and manages environmental data such as temperature, humidity, and room differential pressure in pharmaceutical and medical device manufacturing, testing, and storage areas. This system makes use of Yokogawa paperless recorders that comply with Good Manufacturing Practice (GMP), Quality Management System (QMS), and other regulatory requirements and are installed at multiple locations around a plant. The new system reduces risk and aids pharmaceutical and medical device manufacturers in the performance of strict and efficient environment monitoring that ensures compliance with the various regulations and guidelines on operations at manufacturing sites.
Development Background
In manufacturing, testing, and storage areas at pharmaceutical and medical device plants, the recording and management of environmental data such as temperature, humidity, differential room pressure, and particle count must be done in compliance with GMP, QMS, and other regulations that seek to ensure the correctness, comprehensiveness, completeness, and secure storage of this data. To streamline the management of environmental data measured at multiple locations in a factory, a unified approach is needed. Although data acquisition and storage could be performed by a general-purpose monitoring and control system, such a system would need to be configured to comply with regulatory requirements of various industries. Such a system would also have risk management problems because the data would be collected directly from sensors, and thus any kind of failure, such as power failure, sensor malfunctioning, or recorder capacity exhaustion could affect a broader scope of data. To address such issues, Yokogawa has developed the OpreX Environmental Monitoring System for the pharmaceutical and medical device industries.
![]()
Features
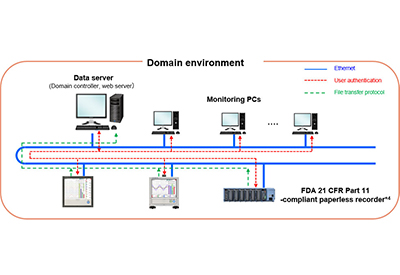
This system consists of Yokogawa paperless recorders, a data server, and monitoring PCs.
- 1. Compliance with regulatory requirements
As required by the FDA’s 21 CFR Part 11 and other electronic recording and signature regulations, the following functions are provided with the OpreX Environmental Monitoring System: a user management function, a function that prevents the rewriting, deletion, or falsification of recorded data files, an audit trail function, a time synchronization function, a backup restoration function, and an archive retrieval function. This ensures that the recorded data is correct, comprehensive, complete, and stored securely. - 2. Data collection and long-term storage
This system’s paperless recorders can be installed at multiple locations around a plant, reducing the risks that come from having all data stored in one central location. The data from the recorders is stored on a highly secure data server using a file format that helps prevent and detect any tampering of data. For backup storage, each recorder is equipped with an SD card function. In response to requests for data by auditors or other individuals, users need only to access and provide the relevant file. - 3. Real-time monitoring
If a data error is detected by a monitoring PC, the PC will display an alarm message, issue an audible alarm, and/or send an email, depending on the paperless recorder setting. In addition, the data that has been collected by a paperless recorder can be accessed from any web browser. - 4. Computerized System Validation (CSV)
The paperless recorders, data server, and monitoring PCs used in this system undergo a CSV assessment that complies with GAMP5*6, a de facto global CSV standard, before shipment to the customer.
Major Target Markets
Pharmaceutical and medical device industries
Applications
- – Recording, storage, monitoring, and management of environmental data, such as temperature, humidity, differential room pressure, particle count, etc. in production, testing, and storage areas
- – Recording, storage, monitoring, and management of temperature and humidity data from freezers and thermo-hygrostats
- – Recording, storage, monitoring, and management of data such as temperature, pressure, etc. for devices used in manufacturing processes
![]()
https://www.yokogawa.com/news/press-releases/2020/2020-10-23/